
Bioburden Testing Market Market CAGR to be at 11.30% By 2032 | Charles River Laboratories International Inc.
Bioburden_Testing_Market1
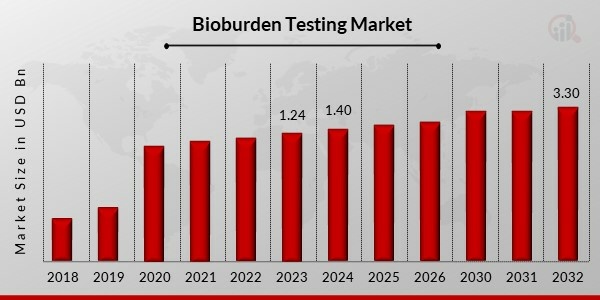
Bioburden testing market projected to grow from $1.40B in 2024 to $3.30B by 2032, fueled by heightened food safety and healthcare demands.
US, NY, UNITED STATES, March 18, 2025 /EINPresswire.com/ -- In the ever-evolving landscape of healthcare safety protocols, bioburden testing has emerged as a critical component ensuring patient safety across medical facilities worldwide. You and I understand the paramount importance of microbial contamination control. Keeping harmful microorganisms at bay protects vulnerable patients.
The Bioburden Testing Market Size was valued at USD 1.24 billion in 2023 and is expected to grow from USD 1.40 billion in 2024 to USD 3.30 billion by 2032, registering a CAGR of 11.30% during the forecast period (2024–2032). The market's expansion is driven by rising food safety concerns, increasing healthcare expenditures, and a surge in product recalls due to microbial contamination.
Download Exclusive Sample Pages Today@ https://www.marketresearchfuture.com/sample_request/6716
Understanding Bioburden Testing in Modern Healthcare
Bioburden testing quantifies viable microorganisms on medical devices. You need this crucial step before sterilization processes. I cannot overstate its importance in healthcare safety.
The testing identifies bacterial presence on medical instruments. You receive actionable data for sterilization protocols. I emphasize this cornerstone of infection prevention protocols.
You might wonder about testing methodologies available today. I can describe various approaches gaining prominence. Traditional culture methods remain widely used techniques.
Rapid microbial detection systems save valuable time. You appreciate faster results in clinical settings. I recognize efficiency drives better patient outcomes.
Key Companies in the Bioburden Testing market include
- Charles River Laboratories International Inc.
- Merck & Co., Inc.
- Lonza
- Becton, Dickinson and Company
- Pacific BioLabs Inc.
- Sotera Health
- North American Science Associates Inc.
- ATS Laboratories
- SGA SA
- Wuxi Pharmatech Inc.
Clinical Applications Driving Market Growth
Hospital-acquired infections pose serious threats to patients. You witness firsthand their devastating impact daily. I understand prevention requires comprehensive testing approaches.
Surgical instruments demand rigorous contamination checks before use. You trust these tools entering patient bodies. I acknowledge this trust requires scientific verification.
Medical device manufacturers implement strict quality controls. You benefit from their commitment to safety. I see how standards protect vulnerable populations.
Pharmaceutical production requires controlled manufacturing environments too. You recognize contamination risks in drug preparation. I appreciate stringent testing ensuring medication safety.
Innovative Technologies Reshaping Bioburden Analysis
Automated testing systems streamline laboratory workflows efficiently. You value consistent results from standardized processes. I notice increased adoption across healthcare facilities.
Rapid detection methods deliver faster actionable results. You implement corrective measures more quickly now. I observe improved patient outcomes from timely interventions.
PCR-based techniques identify specific microbial contaminants precisely. You receive detailed contamination profiles through testing. I recognize how targeted information improves remediation.
Next-generation sequencing revolutionizes contamination source tracking capabilities. You pinpoint problem areas with unprecedented accuracy. I see prevention strategies becoming more effective.
Regulatory Landscape Guiding Testing Protocols
- ISO standards establish minimum testing requirements worldwide
- You must follow these guidelines for compliance
- I emphasize their role in standardizing protocols
- These frameworks ensure consistent patient protection
- FDA regulations govern medical device testing requirements
- You navigate complex regulatory environments daily
- I understand compliance challenges facilities face
- These regulations continually evolve with scientific advances
Purchase Now @ https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=6716
Sustainability Trends in Bioburden Testing
- Environmental considerations influence testing material selection
- You prioritize reduced waste in laboratory settings
- I notice growing adoption of eco-friendly consumables
- Sustainable practices align with broader healthcare goals
- Resource optimization reduces testing environmental footprint
- You implement energy-efficient testing equipment today
- I observe water conservation efforts in laboratories
- These approaches support healthcare sustainability initiatives
Bioburden Testing Market Segmentation
Bioburden Testing Product Outlook
- Consumable
- Instruments
Bioburden Testing End User Outlook
- Pharmaceutical & Biotechnology Company
- Medical Device Manufacturers
- Food & Beverage
- Others
Bioburden Testing Regional Outlook
North America
- US
- Canad
Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- Australia
- Rest of Asia-Pacific
Rest of the World
- Middle East
- Africa
- Latin America
Challenges Facing Bioburden Testing Implementation
Cost concerns remain implementation barriers for facilities. You balance budget constraints with safety needs. I recognize this challenging healthcare equation today.
Time-intensive traditional methods delay critical results sometimes. You seek faster alternatives without sacrificing accuracy. I understand this technological tension exists currently.
Specialized training requirements limit qualified personnel availability. You invest in developing skilled testing teams. I acknowledge education remains crucial for success.
Result interpretation requires experienced microbiological expertise often. You need professionals who understand findings contextually. I see specialized knowledge driving effective responses.
Future Outlook for Bioburden Testing
Artificial intelligence enhances contamination pattern recognition capabilities. You benefit from predictive insights previously unavailable. I anticipate continued algorithm refinement moving forward.
Miniaturized testing devices enable point-of-care applications now. You perform tests closer to patient environments. I observe decentralized testing gaining momentum quickly.
Integration with electronic health records streamlines documentation requirements. You access testing histories seamlessly during procedures. I appreciate workflow improvements enhancing efficiency.
Continuous monitoring systems replace periodic testing approaches. You receive real-time contamination alerts immediately now. I expect this trend to accelerate significantly.
Conclusion: The Path Forward
As we navigate the complex landscape of healthcare safety together, bioburden testing stands as an essential safeguard against dangerous microbial threats. You deserve the highest standards of protection in medical environments. I believe rigorous testing protocols deliver this crucial safety.
You can witness firsthand how proper testing prevents devastating infections. I see the direct connection between laboratory excellence and patient outcomes. Together we acknowledge this vital healthcare component.
You and I recognize that continued innovation will further strengthen our defense against microbial threats. I remain optimistic about technological advances enhancing protection. The future holds promising developments for healthcare safety.
BROWSE MORE HEALTHCARE REPORTS-
Smart Pills Drug Delivery Market - https://www.marketresearchfuture.com/reports/smart-pills-drug-delivery-market-43578
Smart Prosthetic Market - https://www.marketresearchfuture.com/reports/smart-prosthetic-market-43566
Smoking Cessation Aid Market - https://www.marketresearchfuture.com/reports/smoking-cessation-aid-market-43587
Snail Mucin Skincare Market - https://www.marketresearchfuture.com/reports/snail-mucin-skincare-market-36801
Social Determinants Of Health Market - https://www.marketresearchfuture.com/reports/social-determinants-of-health-market-39353
Sodium Hyaluronate Based Product Market - https://www.marketresearchfuture.com/reports/sodium-hyaluronate-based-product-market-43576
Soft Tissue Anchor Market - https://www.marketresearchfuture.com/reports/soft-tissue-anchor-market-39373
Solid Tumor Therapeutic Market - https://www.marketresearchfuture.com/reports/solid-tumor-therapeutic-market-43561
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services.
MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help to answer all their most important questions.
Market Research Future
Market Research Future
+ +1 855-661-4441
email us here
Visit us on social media:
Facebook
X
LinkedIn
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release